ANSI/SLAS Microplate Standards (ANSI/SLAS 1 to 4 – 2004)
The Society For Laboratory Automation And Screening (SLAS) is not a standardizing organization, they are a society that provides a working group the ability and platform to standardize along with the American National Standards Institute (ANSI).
ANSI
The American National Standards Institute is a private, non-profit organization (501 (c)3) that administers and coordinates the U.S. voluntary Standardization and conformity assessment system. “ANSI empowers its members and constituents to strengthen the U.S. marketplace position in the global economy while helping to assure the health of consumers and the protection of the environment.”
ANSI Approval of Microplate Standards
On January 15, 2004, ANSI approved the four proposed standards (listed below) for the design of microplates used in drug discovery and other testing as universal industry standards set forth by the former (SBS) Society of Biomolecular Screening (now known as Society For Laboratory Automation And Screening.)
Original Microplate Standards
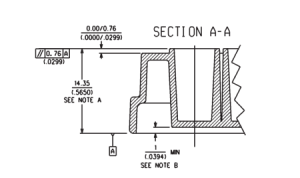
- ANSI/SBS 1-2004: Footprint Dimensions
- ANSI/SBS 2-2004: Height Dimensions
- ANSI/SBS 3-2004: Bottom Outside Flange Dimensions
- ANSI/SBS 4-2004: Well Positions
Internationally Recognized Standards
Prior to the ANSI approval, “vendors hesitated to change their molds until the standards were approved,” noted then SBS Executive Director Christine Giordano, “Now buyers can begin to look for plates that meet internationally recognized standards.” Using microplates that meet a common standard streamlines the process by ensuring compatibility across systems.
Biomolecular Screening
One of the challenges of early stage drug discovery is to accurately identify compounds that can yield effective drugs. Researchers can utilize a variety of automated methods to quickly identify candidate molecules. The microplate plays a critical role in the screening process by allowing a high volume of compounds to be assayed rapidly and efficiently. Microplates commonly used in biomolecular screening will have 96-, 384-, or 1536-wells in which a tiny amount of a compound to be tested is combined with a reagent. The compound’s potential value and effectiveness against a particular target biological system or disease is measured against the subsequent effect on the reagent. Developing a microplate standard ensures compatibility across the variety of automated laboratory instruments used in (HTS) high-throughput screening applications.
ANSI Renews & Renames Existing Microplate Standards 1, 2, 3 and 4
The Society for Laboratory Automation and Screening (SLAS) announced on October 29, 2012 that the American National Standards Institute (ANSI) recently finalized reaffirmation and redesignation of four existing microplate standards¹.
- ANSI/SLAS 1-2004 (R2012) Microplates – Footprint Dimensions (formerly ANSI/SBS 1-2004)
- ANSI/SLAS 2-2004 (R2012) Microplates – Height Dimensions (formerly ANSI/SBS 2-2004)
- ANSI/SLAS 3-2004 (R2012) Microplates – Bottom Outside Flange Dimensions (formerly ANSI/SBS 3-2004)
- ANSI/SLAS 4-2004 (R2012) Microplates – Well Positions (formerly ANSI/SBS 4-2004)
ANSI Accredits New SLAS Microplate Standard for Well Bottom Elevation
On October 9, 2012 the SLAS announced that ANSI recently finalized accreditation of SLAS Microplate Standard 6 for Well Bottom Elevation. According to the SLAS press release, ANSI/SLAS 6-2012 defines terminology and measurement protocols for well bottom elevations (WBE) and well bottom elevation variation (WBEV), and outlines the conditions required for making necessary measurements. The SLAS states “Deviations from these conditions are permissible, provided that the methods used give results in substantial agreement with the methods described in the standard.

Leave a Reply
You must be logged in to post a comment.