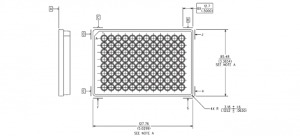
Microplate Standards
The American National Standards Institute (ANSI) and the Society of Biomolecular Screening (SBS) now named the Society For Laboratory Automation And Screening (SLAS) approved a standard for microplates in 2004. In 1995, as early as the first meeting of the SBS, a need for clearly defined dimensional standards of a microplate were identified.
The Need for Standardization
In the mid-90’s the microplate was already becoming an essential tool used in drug discovery research. Before dimensional standards, microplate dimensions varied by manufacturers and even within product lines of individual manufacturers. This variation in dimensions caused a myriad of problems for scientists using microplates in automated laboratory instrumentation.
Timeline: Defining Dimensional Standards
- 1995 – SBS members began working on defining dimensional standards for the standard 96-well microplate. The first written proposal was released in December.
- 1996– The first proposal was presented at numerous scientific conferences and journals throughout the year. Initial proposal was officially presented to the membership of SBS for approval at the annual meeting in October in Basel, Switzerland.
- 1997-1998 – Various versions of the proposed standards for 96- and 384-well microplates were circulated to the membership of the society.
- 1999 – Early in the year, efforts to begin formalizing the proposed standards in preparation for submission to a recognized standards organizations.
Benefits of Standardization
Carol Ann Homon, Co-Chair of SBS Microplate Standards Development Committee (MSDC) stated in a 2004 press release that “Until now, if a scientist ran a screen, he or she had to program the instrument for every microplate. We might have, for example, 100 different types of 96-well microplates, each slightly different from the other.” With the development of microplate standards Homon was quoted as saying “Now we can be sure that if plates meet ANSI/SBS standards, results will be consistent across platforms, and costs to laboratories will be reduced.”
Society of Biomolecular Sciences (SBS)
 In 1994 the Society for Biomolecular Sciences (SBS) was originally founded as the Society of Biomolecular Screening to provide a forum for global education and information exchange among professionals in the chemical, pharmaceutical, biotech, and agrochemical industries¹. In 1995 the Society for Biomolecular Screening (SBS)
In 1994 the Society for Biomolecular Sciences (SBS) was originally founded as the Society of Biomolecular Screening to provide a forum for global education and information exchange among professionals in the chemical, pharmaceutical, biotech, and agrochemical industries¹. In 1995 the Society for Biomolecular Screening (SBS)
Association for Laboratory Automation (ALA)
 The Association for Laboratory Automation (ALA) was a scientific association, organized in 1996, as a nonprofit 501(c)(3) for the medical and biological laboratory automation industry. The ALA’s mission was “to advance science and education related to laboratory automation by encouraging the study, advancing the science, and improving the practice of medical and laboratory automation.” The focus of the ALA was on the benefits and utilization of automation, robotics, and artificial intelligence in order to improve the quality, efficiency, and relevance of laboratory analysis.
The Association for Laboratory Automation (ALA) was a scientific association, organized in 1996, as a nonprofit 501(c)(3) for the medical and biological laboratory automation industry. The ALA’s mission was “to advance science and education related to laboratory automation by encouraging the study, advancing the science, and improving the practice of medical and laboratory automation.” The focus of the ALA was on the benefits and utilization of automation, robotics, and artificial intelligence in order to improve the quality, efficiency, and relevance of laboratory analysis.
SBS and ALA Merger
In 2010 the Society for Biomolecular Sciences and Association for Laboratory Automation merged to form the Society For Laboratory Automation and Screening². The Society For Laboratory Automation and Screening (SLAS) was formed when the two respected and established organizations “agreed that the idea of a merger was viable and attractive.” This standard was processed and approved for submittal to ANSI by the MSDC of the SBS now SLAS.